Research in the Tisdale Lab is focused on the basic science and engineering of energy transport phenomena in nanostructured materials. We care about the mechanisms by which excitons, free charges, phonons (heat), and reactive chemical species are converted to more useful forms of energy, and how we can leverage this understanding to guide materials design and process optimization. Achieving this goal requires information above and beyond what is given by basic characterization, so we develop and implement advanced spectroscopic tools tailored to the scientific problems in which we are interested. Coupling these techniques with control over material synthesis and assembly into thin films allows us to explore the rich physics of nanostructured and quantum-confined materials and gain new insight into energy transport mechanisms in these materials. Read more about current projects and experimental strategies below.
 Materials discovery and design
Materials discovery and design
We synthesize a variety of colloidal nanomaterials including quantum dots and nanoplatelets, and study their self-assembly and material properties. Quantum dots (QDs) are semiconductor crystallites just a few nanometers in diameter, while nanoplatelets are flat structures only a few atomic layers thick by several nanometers in the lateral dimensions. These materials exhibit electronic and optical properties that may be adjusted simply by changing their physical size – the diameter of the nanocrystal or the thickness of the nanoplatelet. The surfaces of both quantum dots and nanoplatelets are covered with long organic molecules called ligands during 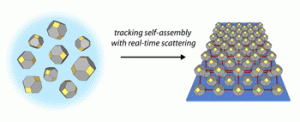 synthesis to make them easily dispersible in organic solvents, but these long ligands are replaced with short organic or inorganic molecules in solid state devices to improve charge transport. Colloidal dispersions are prepared via wet chemistry techniques carried out at moderate temperatures, suggesting a feasible path toward scalable implementation of nanomaterials in next-
synthesis to make them easily dispersible in organic solvents, but these long ligands are replaced with short organic or inorganic molecules in solid state devices to improve charge transport. Colloidal dispersions are prepared via wet chemistry techniques carried out at moderate temperatures, suggesting a feasible path toward scalable implementation of nanomaterials in next-
generation renewable energy technologies like solar power, high efficiency lighting, and thermal energy scavenging.
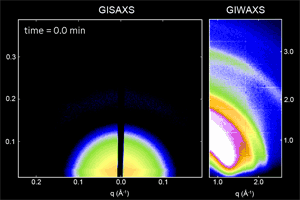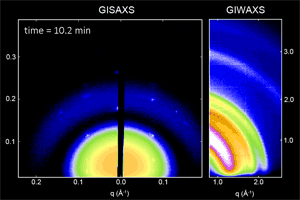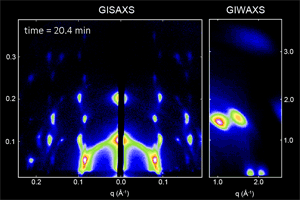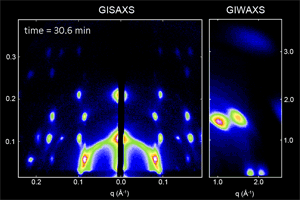
We have developed a synthesis for PbS QDs that allows control of both the QD size and size dispersity, and that is capable of making ensembles in which QDs vary in size by less than one atomic layer (ACS Nano 8,
775 (2014)). We then used x-ray scattering to observe how these monodisperse QDs self-assemble into ordered superlattices (Nat. Mater. 15, 775 (2016)), and how well that order is retained following ligand exchange to reduce inter-particle spacing and improve the transport properties of the quantum dot solid (Chem. Mater. 27, 474 (2015)). We are continuing work in this area to better understand QD properties that facilitate self-assembly into different superlattice crystal structures.
 Ongoing efforts involve expanding our capabilities to other materials systems such as CdSe nanoplatelets, and organometal halide perovskite nanoplatelets and quantum-confined layered structures. Organometal halide perovskites consist of a cubic lattice of metal (often Pb or Sn) and halide (Cl, Br, I) ions, with a freely rotating organic cation (methylammonium or formamidinium) in each unit cell. In addition to tuning the nanoplatelet or layer thickness, the materials properties can also be tuned by composition and choice of ligand (ACS Nano 10, 7830 (2016)). We are actively working to improve the stability of these materials and to explore the physical origins of their unique properties.
Ongoing efforts involve expanding our capabilities to other materials systems such as CdSe nanoplatelets, and organometal halide perovskite nanoplatelets and quantum-confined layered structures. Organometal halide perovskites consist of a cubic lattice of metal (often Pb or Sn) and halide (Cl, Br, I) ions, with a freely rotating organic cation (methylammonium or formamidinium) in each unit cell. In addition to tuning the nanoplatelet or layer thickness, the materials properties can also be tuned by composition and choice of ligand (ACS Nano 10, 7830 (2016)). We are actively working to improve the stability of these materials and to explore the physical origins of their unique properties.
 Charge carrier transport in nanomaterials
Charge carrier transport in nanomaterials
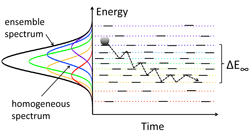
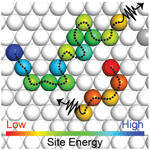
Efficient charge carrier transport is essential for achieving efficient optoelectronic devices such as solar cells and light-emitting diodes made from nanomaterials. Charge carrier transport in nanomaterial-based solids often proceeds via hopping from quantum dot to quantum dot or nanoplatelet to nanoplatelet within the solid. These hopping dynamics are influenced by many factors including the distance between neighboring nanoparticles which is influenced by the surface ligands, the amount of energetic disorder caused by size dispersity and the size-dependent electronic properties, and the extent of structural disorder in the solid. We use a variety of spectroscopic and modeling tools to understand the relationships between material properties and charge transport, with a goal of enabling more efficient nanomaterials-based optoelectronic devices.
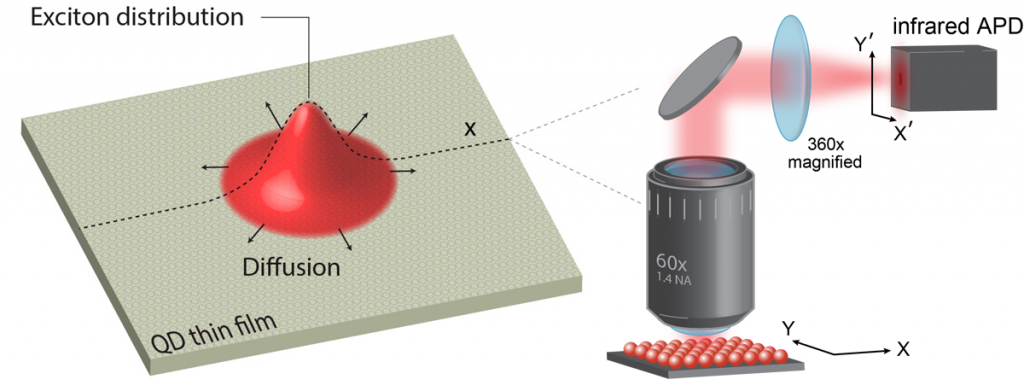 The charge carriers responsible for transport in nanomaterials may be either excitons (bound electron-hole pairs) or free charge carriers depending on the material properties. Photoluminescence occurs when an exciton recombines and emits a photon, so photoluminescence-based techniques make good probes of exciton dynamics. We can directly measure exciton diffusion lengths by using time-resolved optical microscopy to visualize the rate at which the photoluminescence spot size increases following laser excitation (Nano Lett. 14, 3556 (2014)). We also gain further insight into exciton dynamics from temperature-dependent spectrally resolved transient photoluminescence measurements (J. Phys. Chem. C 118, 7894 (2014)) and kinetic Monte Carlo simulations (J. Phys. Chem. B 119, 9501 (2015)). When excitons readily dissociate into free carriers, such as in electronically coupled PbS QD solids, we use transient absorption, which is sensitive to both free carriers and excitons, to monitor carrier dynamics in these materials (Nano Lett. 17, 893 (2017)).
The charge carriers responsible for transport in nanomaterials may be either excitons (bound electron-hole pairs) or free charge carriers depending on the material properties. Photoluminescence occurs when an exciton recombines and emits a photon, so photoluminescence-based techniques make good probes of exciton dynamics. We can directly measure exciton diffusion lengths by using time-resolved optical microscopy to visualize the rate at which the photoluminescence spot size increases following laser excitation (Nano Lett. 14, 3556 (2014)). We also gain further insight into exciton dynamics from temperature-dependent spectrally resolved transient photoluminescence measurements (J. Phys. Chem. C 118, 7894 (2014)) and kinetic Monte Carlo simulations (J. Phys. Chem. B 119, 9501 (2015)). When excitons readily dissociate into free carriers, such as in electronically coupled PbS QD solids, we use transient absorption, which is sensitive to both free carriers and excitons, to monitor carrier dynamics in these materials (Nano Lett. 17, 893 (2017)).
Having developed this powerful set of tools, we are applying them to other materials systems and examining charge carrier loss mechanisms and understanding what limits charge carrier diffusion lengths. Long diffusion lengths allow for the fabrication of thicker and more absorptive/emissive devices. Examples include examining charge carrier trapping dynamics in PbS QD films, and exciton-exciton annihilation in few-layer transition metal dichalcogenides.
Interfacial dynamics
In addition to the active layer that absorbs or emits light, devices also contain layers of other materials to extract charges from or inject charges into the active layer. Efficient charge transfer across the interfaces between the layers is critical for the overall device to function effectively. The unique properties of quantum-confined materials have interesting implications for interfacial charge transfer. We seek to study these phenomena using spectroscopic techniques that are selective for the materials on either side of the interface, or the interface itself, and learn how we can take advantage of nanomaterials to create novel device architectures.
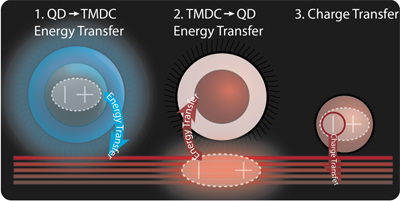 Quantum dots are a material of interest for devices due to their strong absorption and emission, but the hopping transport of charge carriers between quantum dots is relatively slow. Few and single-layer transition metal dichalogenides (TMDCs) have exceptionally good transport properties but weaker emission and absorption. Efficient energy transfer between the two allows for the creation of a hybrid device architecture that takes advantage of the strengths of the two materials while bypassing their weakness. We used time-resolved photoluminescence to observe fast energy transfer between quantum dots and TMDCs. Interestingly, energy transfer was faster to TMDC regions with fewer layers due to leakage of the dipole electric field into the surrounding medium and donor QDs (Nano Lett. 14, 6087 (2014)).
Quantum dots are a material of interest for devices due to their strong absorption and emission, but the hopping transport of charge carriers between quantum dots is relatively slow. Few and single-layer transition metal dichalogenides (TMDCs) have exceptionally good transport properties but weaker emission and absorption. Efficient energy transfer between the two allows for the creation of a hybrid device architecture that takes advantage of the strengths of the two materials while bypassing their weakness. We used time-resolved photoluminescence to observe fast energy transfer between quantum dots and TMDCs. Interestingly, energy transfer was faster to TMDC regions with fewer layers due to leakage of the dipole electric field into the surrounding medium and donor QDs (Nano Lett. 14, 6087 (2014)).
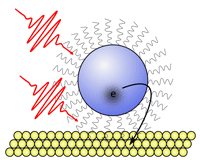 For looking at interfacial dynamics on shorter time scales, we are implementing a surface-selective ultrafast spectroscopy technique, second harmonic generation (SHG). SHG is ideal for probing surfaces and interfaces because it is forbidden in centrosymmetric materials, and inversion symmetry is generally broken at surfaces and interfaces. SHG is also sensitive to electric fields making it a powerful tool for detecting charge transfer across interfaces over picosecond or sub-picosecond time scales. SHG has been a focus for technique development in our lab, and we have implemented a scheme to boost its intrinsically low signal levels using optical stimulation (Phys. Rev. Lett. 114, 183902 (2015)).
For looking at interfacial dynamics on shorter time scales, we are implementing a surface-selective ultrafast spectroscopy technique, second harmonic generation (SHG). SHG is ideal for probing surfaces and interfaces because it is forbidden in centrosymmetric materials, and inversion symmetry is generally broken at surfaces and interfaces. SHG is also sensitive to electric fields making it a powerful tool for detecting charge transfer across interfaces over picosecond or sub-picosecond time scales. SHG has been a focus for technique development in our lab, and we have implemented a scheme to boost its intrinsically low signal levels using optical stimulation (Phys. Rev. Lett. 114, 183902 (2015)).
Thermal transport
Efficient heat dissipation is critical for devices to function efficiently, but quantum dot films have a much lower thermal conductivity than bulk semiconductors. Thermal energy is stored as vibrations in an atomic lattice or molecule, known as phonons. QD films are composite materials, consisting of inorganic cores with organic ligands bound to the surface. Engineering these materials for superior thermal properties requires a full understanding of how both parts of the structure and the interface between them contributes to the QD’s vibrational structure and which phonons are responsible for dissipating excess thermal energy.
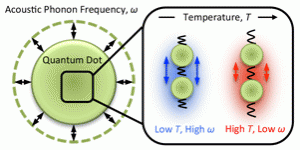 Vibrational spectroscopy techniques developed in our lab allow us to observe these vibrations. One particular set of vibrations thought to be important for thermal conductivity are low frequency acoustic modes in the QD core. These are long-range deformations in the atomic lattice such as the symmetric expansion and contraction of the entire nanocrystal, called the radial breathing mode. We have used a specialized low-frequency Raman spectroscopy technique to measure how changing the surface ligands (J. Phys. Chem. Lett. 7, 4213 (2016)) and the film temperature (Phys. Chem. Chem. Phys. 18, 28797 (2016)) affect this radial breathing mode, and used the data to accurately model the nanocrystal as an elastic sphere.
Vibrational spectroscopy techniques developed in our lab allow us to observe these vibrations. One particular set of vibrations thought to be important for thermal conductivity are low frequency acoustic modes in the QD core. These are long-range deformations in the atomic lattice such as the symmetric expansion and contraction of the entire nanocrystal, called the radial breathing mode. We have used a specialized low-frequency Raman spectroscopy technique to measure how changing the surface ligands (J. Phys. Chem. Lett. 7, 4213 (2016)) and the film temperature (Phys. Chem. Chem. Phys. 18, 28797 (2016)) affect this radial breathing mode, and used the data to accurately model the nanocrystal as an elastic sphere.
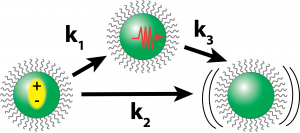 Ongoing efforts in our lab are approaching thermal transport from two directions. First, we are employing femtosecond stimulated Raman scattering (FSRS) to measure transient changes in the vibrational structure of a nanoparticle after excitation by a laser pulse. FSRS will allow us to observe the movement of vibrational energy around the structure and the amount of time it takes for heat to cross the organic/inorganic interface. We are also developing a method to accurately measure the thermal conductivity of thin films using frequency domain thermoreflectance enabling us to translate how microscopic observations translate to changes in macroscopic thermal conductivity.
Ongoing efforts in our lab are approaching thermal transport from two directions. First, we are employing femtosecond stimulated Raman scattering (FSRS) to measure transient changes in the vibrational structure of a nanoparticle after excitation by a laser pulse. FSRS will allow us to observe the movement of vibrational energy around the structure and the amount of time it takes for heat to cross the organic/inorganic interface. We are also developing a method to accurately measure the thermal conductivity of thin films using frequency domain thermoreflectance enabling us to translate how microscopic observations translate to changes in macroscopic thermal conductivity.